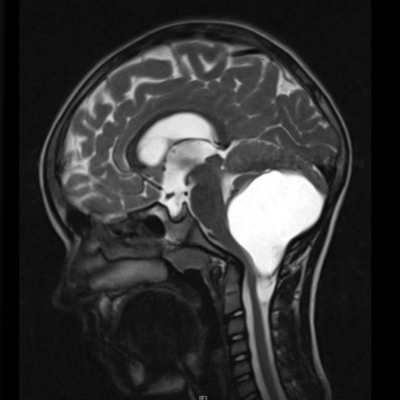
The LOGGIC Core BioClinical database (LOGGIC Core) is a registry of molecular and clinical data for pediatric low-grade gliomas (LGG). Since early 2019, patients have been recruited from an ever-increasing number of participating countries within and outside Europe.
Background information about low-grade gliomas
Low-grade gliomas (LGG) are a heterogeneous group of central nervous system (CNS) tumors classified as grade 1 and 2 tumors by the World Health Organization (WHO). The 10-year survival rate is over 90% and the probability of 10-year event-free survival is approximately 45%. This means that many patients suffer recurrent relapses and often receive multiple therapies. Many patients with LGG are only followed up closely after initial diagnosis or primary tumor resection. However, in several patients, the tumor is not resectable or not completely resectable and/or radiologically progressive and/or concurrent clinical symptoms are present. These chronically ill patients require - sometimes multiple - surgeries and additional non-surgical therapy.
Objectives
LOGGIC Core data will be used to improve understanding of the tumor biology of the disease and to correlate molecular subsets of LGG with clinical courses, as well as to direct patients with tumors exhibiting appropriate molecular alterations to appropriate clinical trials.
Specifically, the database is expected to achieve the following goals:
- Increase knowledge about genetic characteristics of LGGs.
- Identification of targets that are potentially useful for therapies
- Identification of biomarkers that can more accurately predict disease progression and response to chemo- and targeted therapies
- Establishment of novel LGG tumor models to develop new therapeutic approaches
At KiTZ
Dr. David T. W. Jones
Head of the Department of Pediatric Glioma Research
Hopp Children's Tumor Center Heidelberg (KiTZ)
German Cancer Research Center (DKFZ)
Prof. Dr. Till Milde, MD
Senior physician - KiTZ Clinical Trial Unit
Group leader - Translational brain tumor modeling, KiTZ
KKE Pediatric Oncology, DKFZ
Department of Pediatric Oncology, Hematology, Immunology and Pulmonology, Heidelberg University Hospital
Prof. Dr. Felix Sahm, MD und Prof. Dr. Andreas von Deimling, MD
Clinical Cooperation Unit (CCU) Neuropathology
Department of Neuropathology at the Institute of Pathology,
Heidelberg University Hospital
Dr. med. Kristian W. Pajtler, MBA
Group leader – Early Cancer Diagnostics & Reverse Translation, KiTZ
Division of Pediatric Neurooncology, DKFZ
Department of Pediatric Oncology, Hematology, Immunology and Pulmonology, Heidelberg University Hospital
At Charité
Prof. Dr. David Capper, MD
Department of Neuropathology
Charité - Universitätsmedizin Berlin
PD Dr. Arend Koch, MD
Department of Neuropathology
Charité - Universitätsmedizin Berlin
Project management
Dr. Carina Bodden
KiTZ Clinical Trial Unit
Hopp Children's Tumor Center Heidelberg (KiTZ)
German Cancer Research Center (DKFZ)
Heidelberg University Hospital
Data management
Central Data Management of the Society for Oncology and Hematology (GPOH ZDM gGmbH)
c/o Hannover Medical School
Pediatric Oncology/Hematology
Funding
The Brain Tumour Charity (UK): The EVEREST Centre for Paediatric Low-Grade Brain Tumour Research
Cooperation partner
On an international level, there is a close cooperation with the SIOPE-BTG and on a national level with the national HIT LOGGIC Registry at the Charité-Universitätsmedizin Berlin for children and adolescents with low-grade gliomas. LOGGIC Core provides biological data that is used together with central clinical data to allow patients to enter (future) phase I/II/III trials.