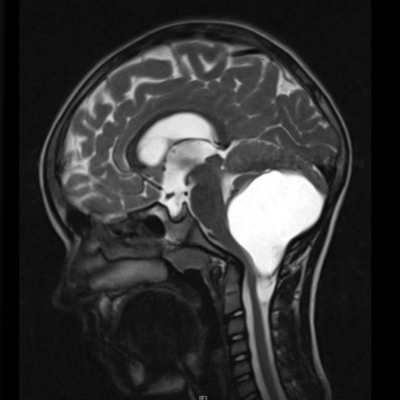
The EVEREST consortium for Low-grade Paediatric Brain Tumours, led by David Jones, division head at the Hopp Children’s Cancer Center Heidelberg (KiTZ) and German Cancer Research Center (DKFZ), receives another 5.8 million euros funding for its research on low-grade gliomas (pLGG) in children.
Pediatric low-grade glioma is an understudied tumor type in children despite accounting for almost half of all childhood brain tumors. In Germany and the UK combined, more than 400 children and adolescents are diagnosed every year. These tumors are typically benign and have usually good survival rates, yet children face lifelong effects from the location of the tumor of the treatment, significantly affecting the quality of life for these children and their families. The ultimate goal of the EVEREST program is to maintain high cure rates while improving the quality of survivorship.
Since its start in 2017, the EVEREST consortium, also encompassing experts from the University College London (UCL) and the Charité in Berlin, has made close partnership with the leading pediatric low-grade program at the Dana Faber Cancer Institute and Boston Children’s Hospital in the USA. The research of the consortium focuses on understanding the biology of pLGG, including the use of cutting-edge technology to better diagnose these tumors, and to develop more effective and less toxic treatment strategies. One of the major accomplishments of EVEREST has been the contribution towards new diagnostic standards for childhood brain tumors, which have been incorporated in the latest edition of the WHO Classification of the Central Nervous System Tumors.
Future goals and projects include the use of new artificial intelligence computing tools to help analyse microscope images of brain tumour samples, a potential rapid technique that could be applied worldwide. With the collection of data from thousands of individual tumors, the team will apply the latest methods to investigate differences between tumor cell types and the interaction with tumor cells and normal cells in unprecedented detail. Another focus will be on pre-clinical laboratory model development and the usage of the models established during the first funding period, including a cell-based system to track the activity of a key signalling molecule within the cells. These models will allow for the screening of new targets that can block the tumor’s growth and reveal differences in how the models respond to different treatments. The clinical trial part of the consortium focuses on novel trial concepts that derive from results decribed above to address the many challenges in the clinical management of pediatric low-grade brain tumors. An important part of the program is to improve quality of life for the patients by reducing side-effects. This will be assessed by a variety of tests and the use of advanced digital monitoring in form of smartphone apps and/or wearable devices to capture challenges of the disease in everyday life, to improve disease management for patients and their families.