Translational pediatric radio-oncology
The junior research group "Translational Pediatric Radiation Oncology" led by Dr. Maximilian Deng is part of the KiTZ and the University Hospital Heidelberg. The focus of the research group is the integration of new molecular predictors into clinical decision making, enabling a personalized, tumor biology-adapted radiation treatment planning and target volume definition. In close collaboration with the preclinical program of the KiTZ, the junior research group aims to identify mutations associated with radioresistance, with the subsequent aim of addressing these alterations therapeutically through combination therapies.
The objective of the junior research group is to establish innovative and personalized radiation treatment concepts for childhood tumors, ultimately improving the long-term outcome while minimizing treatment-associated side effects of our young patients.

Spotlight - JRG Deng
Molecular tumor profiling facilitates personalized radiation treatment planning
Recent large-scale genomic profiling has improved our understanding of the diverse molecular landscape of various pediatric cancer types. Our research aims to translate the molecular heterogeneity in pediatric cancer types as an additional stratum in clinical decision-making during radiation treatment planning.
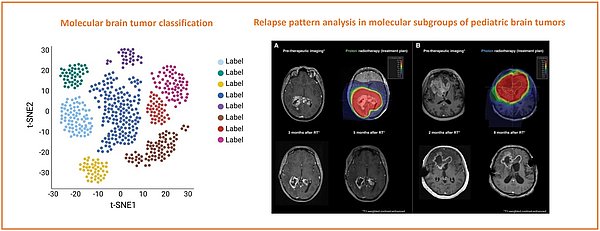
Pediatric brain tumors are the leading cause of cancer-related death in children. Recent advances in genomic profiling have revolutionized our understanding of these tumors, revealing distinct molecular subgroups in tumor entities, which were previously thought to represent homogenous diseases. Despite significant progress in the past decades in the management of pediatric brain tumors, tumor relapse remains a critical challenge, particularly for high-risk subgroups. Relapse patterns can vary considerably among different molecular subgroups, reflecting underlying differences in tumor biology and response to therapy. Understanding these patterns is essential for improving therapeutic decision making in radiation oncology (e.g., target volume definition). By leveraging comprehensive genomic data and clinical follow-up information, we seek to identify subgroup-specific relapse dynamics. Our findings will contribute to the growing body of knowledge on the molecular underpinnings of pediatric brain tumors and inform the development of personalized treatment approaches during radiation treatment planning, ultimately with the hope to improve survival in this vulnerable population.
Reverse Translation: post-hoc identification of putative alterations associated with radioresistance
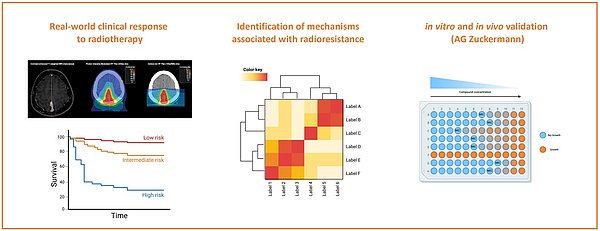
Radiation therapy represents a cornerstone in the treatment of various cancers, yet its effectiveness is often compromised by the emergence of radioresistant tumor cells. To address this challenge, we utilize a reverse translation approach to identify putative genetic and epigenetic alterations associated with radioresistance. By retrospectively analyzing clinical data alongside molecular profiles from treated patients, we aim to pinpoint specific alterations conferring resistance to radiation therapy. Our work aims to integrate comprehensive genomic data with clinical outcomes to uncover potential biomarkers and therapeutic targets for personalized treatment strategies. In collaboration with the Preclinical Program at KiTZ (AG Zuckermann), in vivo models will be generated to test and target these putative alterations, providing a robust platform for validating our findings and advancing the development of combination therapy. Our research enhances the understanding of radioresistance mechanisms and demonstrates the utility of reverse translation in identifying clinically relevant genetic alterations, ultimately aiming to improve therapeutic outcomes and patient survival.
Efficacy and toxicity of bimodal radiotherapy in WHO grade 2 meningiomas following subtotal resection with carbon ion boost: Prospective phase 2 MARCIE trial. Deng MY, Maas SLN, Hinz F, Karger CP, Sievers P, Eichkorn T, Meixner E, Hoegen-Sassmannshausen P, Hörner-Rieber J, Lischalk JW, Seidensaal K, Bernhardt D, Jungk C, Unterberg A, Wick A, Wick W, von Deimling A, Sahm F, Combs S, Herfarth K, Debus J, König L. Neuro Oncol. 2024 Apr 5;26(4):701-712. doi: 10.1093/neuonc/noad244. PMID: 38079455; PMCID: PMC10995516.
Analysis of recurrence probability following radiotherapy in patients with CNS WHO grade 2 meningioma using integrated molecular-morphologic classification. Deng MY, Hinz F, Maas SLN, Anil G, Sievers P, Conde-Lopez C, Lischalk J, Rauh S, Eichkorn T, Regnery S, Bauer L, Held T, Meixner E, Lang K, Hörner-Rieber J, Herfarth K, Jones D, Pfister SM, Jungk C, Unterberg A, Wick W, von Deimling A, Debus J, Sahm F, König L. Neurooncol Adv. 2023 May 14;5(1):vdad059. doi: 10.1093/noajnl/vdad059. PMID: 37293256; PMCID: PMC10246580.
Radiation-induced gliomas represent H3-/IDH-wild type pediatric gliomas with recurrent PDGFRA amplification and loss of CDKN2A/B. Deng MY, Sturm D, Pfaff E, Sill M, Stichel D, Balasubramanian GP, Tippelt S, Kramm C, Donson AM, Green AL, Jones C, Schittenhelm J, Ebinger M, Schuhmann MU, Jones BC, van Tilburg CM, Wittmann A, Golanov A, Ryzhova M, Ecker J, Milde T, Witt O, Sahm F, Reuss D, Sumerauer D, Zamecnik J, Korshunov A, von Deimling A, Pfister SM, Jones DTW. Nat Commun. 2021 Sep 20;12(1):5530. doi: 10.1038/s41467-021-25708-y. PMID: 34545083; PMCID: PMC8452680.
Diffuse glioneuronal tumour with oligodendroglioma-like features and nuclear clusters (DGONC) - a molecularly defined glioneuronal CNS tumour class displaying recurrent monosomy 14. Deng MY, Sill M, Sturm D, Stichel D, Witt H, Ecker J, Wittmann A, Schittenhelm J, Ebinger M, Schuhmann MU, Figarella-Branger D, Aronica E, Staszewski O, Preusser M, Haberler C, Lauten M, Schüller U, Hartmann C, Snuderl M, Dunham C, Jabado N, Wesseling P, Deckert M, Keyvani K, Gottardo N, Giangaspero F, von Hoff K, Ellison DW, Pietsch T, Herold-Mende C, Milde T, Witt O, Kool M, Korshunov A, Wick W, von Deimling A, Pfister SM, Jones DTW, Sahm F. Neuropathol Appl Neurobiol. 2020 Aug;46(5):422-430. doi: 10.1111/nan.12590. Epub 2020 Feb 5. PMID: 31867747.
Molecularly defined diffuse leptomeningeal glioneuronal tumor (DLGNT) comprises two subgroups with distinct clinical and genetic features. Deng MY, Sill M, Chiang J, Schittenhelm J, Ebinger M, Schuhmann MU, Monoranu CM, Milde T, Wittmann A, Hartmann C, Sommer C, Paulus W, Gärtner J, Brück W, Rüdiger T, Leipold A, Jaunmuktane Z, Brandner S, Giangaspero F, Nozza P, Mora J, Morales la Madrid A, Cruz Martinez O, Hansford JR, Pietsch T, Tietze A, Hernáiz-Driever P, Stoler I, Capper D, Korshunov A, Ellison DW, von Deimling A, Pfister SM, Sahm F, Jones DTW. Acta Neuropathol. 2018 Aug;136(2):239-253. doi: 10.1007/s00401-018-1865-4. Epub 2018 May 15. PMID: 29766299.